
COVID shots for young kids arrived in June. Few have received them.
Doctors and public health experts never expected there would be so little interest in vaccines for young children.

Doctors and public health experts never expected there would be so little interest in vaccines for young children.

The federal No Surprises Act—which took effect Jan. 1 and protects patients from receiving surprise medical bills resulting from unexpected, out-of-network coverage—is already creating huge waves.

In recent months, current and former employees of drugmaker Eli Lilly and Co., medical-equipment maker Roche Diagnostics and health care system Ascension St. Vincent have filed suit in federal district court, claiming their religious views and civil liberties were violated.

The federal government has purchased more than 170 million doses of the updated boosters, and doses began shipping last week, following authorization by the Food and Drug Administration.

CDC’s advisers deemed the updated injections the best option considering the U.S. still is experiencing tens of thousands of COVID-19 cases and about 500 deaths every day—even before an expected new winter wave.

Prevounce Health already had two remote employees here, and the central location and business-friendly climate were appealing. The company moved here in October, taking office and warehouse space on Post Road.
Pfizer asked U.S. regulators Monday to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives—a key step toward opening a fall booster campaign.

VoCare, which makes a handheld gadget called Vitals360 that allows physicians to monitor their patients’ vital signs remotely, is in the middle of a nasty fight with a group of five early investors.
The commission said more money is needed because the state ranks among the worst in the nation for obesity, smoking, infant mortality and other critical measures, and the life expectancy of Hoosiers has declined in recent years.
An Indiana House committee made significant changes Tuesday to the Republican-backed bill that would ban virtually all abortions in the state.
A startup company that specializes in buying written-off medical accounts is suing a Connecticut insurer for $15.1 million, claiming it failed to fully pay St. Vincent Emergency Physicians for services it provided to its members. Ecure Indiana Corp. filed suit July 28 against United Healthcare Insurance Co.
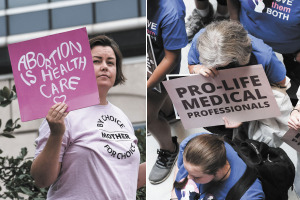
The clinics are in the crosshairs of the Indiana General Assembly and are likely to face a sharp drop-off in business if current legislation passes that would significantly restrict access to abortion.
The research, published online Tuesday by the journal Science, shows that the Huanan Seafood Wholesale Market was likely the early epicenter of the scourge that has now killed nearly 6.4 million people around the world.
Indiana-based pharmaceutical testing company Inotiv Inc. disclosed late Monday that it expects to incur charges of between $7.4 million and $9.9 million for the previously announced shutdowns of two Virginia animal-breeding facilities.
As the Legislature prepares to convene for a special session to consider abortion-related legislation, some doctors are worried about possible criminal liability they might face for providing abortions, even to save the life of the mother.
The unveiling of the proposal ends weeks of speculation on how restrictive the proposal would be, following the U.S. Supreme Court’s decision to turn such matters over to the states. The Indiana Legislature will convene in special session on Monday to consider the legislation.

Novavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S.—and one that’s already available in Europe and multiple other countries.
The surge reversed years of progress fighting one of the gravest public health challenges in modern medicine, according to a new analysis released Tuesday by the Centers for Disease Control and Prevention.
Officials warn of a possible fall or winter wave—perhaps as many as 100 million infections in the United States—that could again flood hospitals with COVID patients.
The FDA is considering ordering a recipe change for the vaccines made by both Pfizer and rival Moderna in hopes that modified boosters could better protect against another COVID surge expected this fall and winter.