FDA issues warning to local doctor for breaking protocol on drug trial
The federal agency says the Indianapolis doctor studying Pfizer’s Chantix last year failed to keep accurate records and used patients who didn’t meet the trial requirements.
The federal agency says the Indianapolis doctor studying Pfizer’s Chantix last year failed to keep accurate records and used patients who didn’t meet the trial requirements.
Vice President Mike Pence may have just picked another fight with pharmaceutical companies—one that doesn’t involve drug prices.
One of the bill’s author said it is designed to help parents who are “up against a wall,” and he stressed that it should not be confused as a first step to medical marijuana legalization in the state.
A small Carmel-based biotech firm has signed a deal with international drug company Allergan Plc that is worth at least $50 million and could grow to more than $2 billion under the best-case scenario.
Research developed at Purdue University and licensed by a biomedical startup could give patients with late-stage prostate cancer an alternative to hormone therapies that can develop resistance after prolonged use.
The National Institute on Aging is awarding $25 million to the Alzheimer's Disease Precision Models Center, a joint project of the Indiana University School of Medicine in Indianapolis and The Jackson Laboratory in Bar Harbor.
The Indiana University School of Medicine got the donation from the children of Indianapolis real estate developer Sidney Eskenazi. The endowed fund will be used to recruit a cancer researcher to Simon Cancer Center.
The planned demolitions of the old IUPUI Psychiatric Research Building and the Wishard Helipad site are the next projects sparked by the land swap between IUPUI and Eskenazi Health’s parent.
The money will be awarded from IU’s Grand Challenges Program, a new push that is designed to tackle “major and large-scale problems facing humanity” that can only be addressed by multidisciplinary research teams.
Animated Dynamics Inc. has raised $1.7 million in early funding to help it get its technology to market.
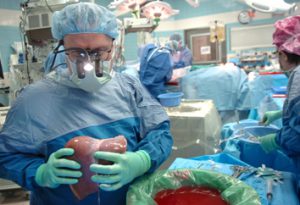
Dr. Joseph Tector, who built IU Health’s transplant program into one of the nation’s largest before announcing his departure Friday, is seeking back wages and penalties worth $4.7 million from the hospital system.
The Indiana Biosciences Research Institute, set up just three years ago, announced Wednesday morning that it has been awarded grants of $80 million from the Lilly Endowment and $20 million from the Eli Lilly and Co. Foundation.

A professor in the Indiana School of Medicine is hopeful that an antibiotic cocktail he invented will one day improve the lives of millions of people, thanks in part to the Indiana University Research and Technology Corp., formed in 1997 to make work done by IU faculty and researchers available for commercial development.

City leaders want to make the 60-acre tract of land just north of the Indiana University School of Medicine campus a mix of all of the best the city has to offer and catch the eyes of more creative and highly sought-after workers.
Researchers at the Indiana University School of Medicine found that blocking the activity of a key protein can significantly cut down on the graft-versus-host immune response suffered by large numbers of patients who receive stem cell transplants.

The drugmaker is trying to beef up its work on using the immune system to fight cancer. Indianapolis will remain Lilly’s main hub for research and development.

The new head of research at the Indiana University School of Medicine thinks the institution is missing out on the more than $6 billion spent each year in the United States on clinical trials.

Michael A. Byers’ Tooth Bank is one of a tiny group of U.S. companies catering to the latest iteration of stem cell therapy: harvesting stem cells from the pulp inside baby teeth and extracted wisdom teeth, then culturing, freezing and storing them at a cryostorage facility for later use.
Dr. Keith March at the IU School of Medicine is almost like a medical superhero, churning out patents at warp speed.
Researchers at Purdue University, the Indiana University School of Medicine and the University of Wisconsin discovered a combination of two currently available drugs significantly slowed the growth of late-stage prostate cancer tumors in mice.