
Contract drugmaker Catalent shrinks as pandemic wanes
The New Jersey-based company has announced two large rounds of layoffs at its Bloomington plant within six months—400 workers last December and 150 this month.

The New Jersey-based company has announced two large rounds of layoffs at its Bloomington plant within six months—400 workers last December and 150 this month.

A growing roster of corporate and political foes has started to lay siege to the law, hoping to erode some of its key provisions before they can take effect.

This year is the second year Indiana has received money from a national settlement with opioid distributors and manufacturers for opioid-related harms.

Indianapolis-based Elevance Health, which operates Anthem plans, said that in most cases, it won’t cover Ozempic unless a patient is diagnosed with diabetes and has tried another medication to manage it, but physicians can still prescribe it.

The Indianapolis-based pharmaceutical giant is spending hundreds of millions of dollars to win over patients with an extensive advertising campaign.

Groups such as the Alzheimer’s Association have pushed Medicare to cover the new Alzheimer’s drugs—including those cleared on an expedited basis—saying that the FDA should be the final arbiter of safety and efficacy of drugs.

Under the agreement, Lilly has agreed to continue its cap on out-of-pocket costs for its users at $35 a month for four years.

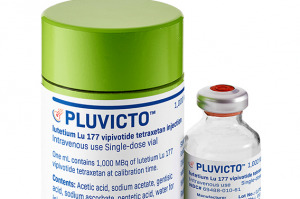
The Indianapolis-based radiopharmaceutical company said it plans to help open a facility in Belgium to make therapeutic agents for cancer patients.
Senate Enrolled Act 8 is part of a sweeping effort this year by the Indiana General Assembly to bring down the cost of health care across the state, where prices are among the highest in the country.

Eli Lilly and Co. shares have soared 57% over the past 52 weeks, buoyed in part by sales of its diabetes drug Mounjaro, which the company is also preparing to seek approval to market for obesity.

If approved, Donanemab has annual multi-billion-dollar sales potential. Lilly shares rose 6% in early-afternoon trading.
There were 301 active national drug shortages through this year’s first quarter, according to the University of Utah Drug Information Service. That’s 49% higher than the 202 recorded in the first three months of 2018.

If approved for weight loss, Eli Lilly’s tirzepatide could become the most effective drug to date in an arsenal of medications that are transforming the treatment of obesity, which affects more than 4 in 10 American adults and is linked to dozens of diseases.
Purdue University plans to use the funding to further support research at the university.
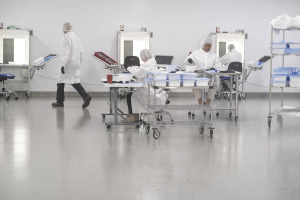
Fishers-based INCOG Biopharma Services Inc., one of the newest players in the $132 billion contract drug manufacturing industry, acts as a behind-the-scenes player for companies that need to get sterile injectable medicines to market in a hurry.
The court extended women’s access to an abortion pill until Friday while the justices consider whether to allow restrictions on mifepristone to take effect as a legal challenge to the medication’s Food and Drug Administration approval continues.

The expansion will bring Eli Lilly and Co.’s total investment in the project to $3.7 billion, the most the company has ever spent on a single manufacturing site.

The Indiana House bill is key to providing women quicker access to contraceptives, bill sponsor Republican Sen. Sue Glick said Tuesday, especially in areas where they struggle to receive primary care.
A federal judge in Texas blocked U.S. government approval of a key abortion medication Friday, siding with abortion foes in an unprecedented lawsuit and potentially upending nationwide access to the pill widely used to terminate pregnancies.

A pharmacist convicted of producing and distributing adulterated drugs was not entitled to attorney fees in his case against the Indiana Board of Pharmacy, which was entitled to immunity, the Court of Appeals of Indiana has ruled.