
Purdue lands $20M gift to develop leaders for pharmacy industry
The donation comes from the former CEO of Santa Barbara Specialty Pharmacy, which services California and five nearby states.

The donation comes from the former CEO of Santa Barbara Specialty Pharmacy, which services California and five nearby states.

The Lebanon Plan Commission voted unanimously Monday to approve Eli Lilly and Co.’s development plan for its two manufacturing facilities in Boone County.
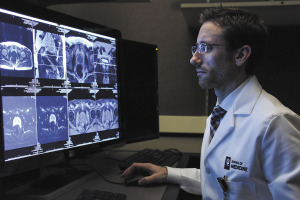
Illuccix was first approved by the FDA in December 2021. The imaging agent is designed to target and “light up” cancerous cells, making it easier for oncologists to determine through a PET scan if the cells have spread to areas outside the prostate.

The medication has been used by millions of Americans since the FDA granted it emergency use authorization in late 2021.

Eli Lilly said the study concludes its clinical development of solanezumab, apparently shutting the door on one of the most closely watched experimental drugs over the past decade.

The decision comes after Attorney General Todd Rokita and 19 other attorneys general threatened legal action if the pharmacy company sells the pills by mail.

The Weinberg Center for Corporate Governance at the University of Delaware, a leading voice for responsible corporate leadership, has generally supported the elimination of staggered terms, saying they can lead to entrenched boards and management that fails to perform.

Shares in the animal health care company have lost more than two-thirds of their value in the past 18 months, but Elanco says a bevy of new products in its pipeline will prove an era of strong growth is yet to come.

The Indianapolis-based drugmaker also said it will expand a program that caps patient out-of-pocket costs at $35 or less a month.
The potential move represents the latest government effort to increase use of a medication that has been a key tool in the battle against the U.S. overdose epidemic that kills more than 100,000 people annually.

Jaypirca was approved to treat mantle cell lymphoma, or MCL. a rare blood cancer that starts in white blood cells in the lymph nodes for which there is no cure, according to Lilly. The disease affects about 1 in 200,000 people worldwide each year.

Donanemab’s ability to rapidly remove amyloid beta from patients’ brains prevented the required number of patients from receiving the drug for a full 12 months, Lilly said, resulting in the FDA rejection.
The Indianapolis company, located in the former St. Bernadette Catholic Church on the near-east side, plans to make the hires by the end of 2025.

California on Thursday announced it will sue the companies that make and promote most of the nation’s insulin, accusing them of scheming to illegally increase the price of the drug.
It’s the first drug that’s been convincingly shown to slow the decline in memory and thinking that define the disease. But the medication comes with downsides, including potentially serious side effects and the need for frequent drug infusions.

The Indianapolis-based pharmaceutical company is preparing for one of its most ambitious years in memory for 2023, with plans to advance five new drugs into late-stage clinical studies and move four into regulatory review.
CVS and Walgreens have agreed to pay state and local governments a combined total of more than $10 billion to settle lawsuits over the toll of opioids.
The report comes in the wake of heavy criticism of the agency’s handling of a formula shortage earlier this year. Food safety experts have long complained that the agency’s food oversight arm has been chronically understaffed and underfunded.
In an amazing resurrection, teplizumab, developed by another company after Lilly trials were a letdown, is one of the hottest new drugs on the market.

Months after Indiana’s attorney general said he’d send about 660 local governments their shares of the state’s $507 million opioid crisis settlement with drug manufacturers and distributors, none have received the money.