
Health care costs among top Indiana legislative priorities
Hospitals are expected to come under more scrutiny and public health spending will be debated in this year’s Indiana General Assembly.

Hospitals are expected to come under more scrutiny and public health spending will be debated in this year’s Indiana General Assembly.

Tuesday’s announcement comes as 3M is facing an onslaught of lawsuits from states and individuals who are claiming contamination from PFAS harmed their health.

The former chair of the Senate Appropriations Committee will testify before that same committee to ask lawmakers to allocate an additional quarter-of-a-billion dollars annually toward public health programs.
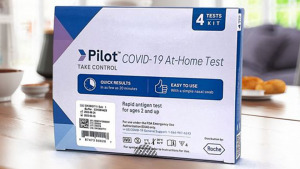
The test is the first over-the-counter test distributed in the U.S. by Roche Diagnostics, which has its North American headquarters in Indianapolis.

A former IndyGo bus could start a second life by the end of the year—distributing fresh food, providing nutrition education and troubleshooting problems Indianapolis residents have applying for food stamps.

The FDA cleared the COVID-19 booster tweaks without requiring human test results—just like it approves yearly changes to flu vaccines.
Between 7 million and 23 million Americans—including 1 million who can no longer work—are suffering from the long-term effects of infection with the virus, according to government estimates.

Host Mason King talks with Dr. Cameual Wright and Jack E. Turman Jr. about the Housing Equity for Infant Health Initiative, a program that will provide support for pregnant women and mothers with infants under 1 year old.

Indianapolis and Denver have been selected as two cities that will work with the Maryland-based Partnership for a Healthier America and the International Fresh Produce Association, a Delaware-based trade group, to try to double residents’ consumption of fruit and vegetables.

The new estimate is a dramatic increase from the roughly $16 billion in potential fraud identified a year ago, and it illustrates the immense task still ahead of Washington as it seeks to pinpoint the losses, recover the funds and hold criminals accountable.
Sharply rising cases of some sexually transmitted diseases—including a 26% rise in new syphilis infections reported last year—are prompting U.S. health officials to call for new prevention and treatment efforts.

Doctors and public health experts never expected there would be so little interest in vaccines for young children.

The federal government has purchased more than 170 million doses of the updated boosters, and doses began shipping last week, following authorization by the Food and Drug Administration.

CDC’s advisers deemed the updated injections the best option considering the U.S. still is experiencing tens of thousands of COVID-19 cases and about 500 deaths every day—even before an expected new winter wave.
Pfizer asked U.S. regulators Monday to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives—a key step toward opening a fall booster campaign.
The changes are driven by a recognition that an estimated 95% of Americans 16 and older have acquired some level of immunity, either from being vaccinated or infected, agency officials said.
The commission said more money is needed because the state ranks among the worst in the nation for obesity, smoking, infant mortality and other critical measures, and the life expectancy of Hoosiers has declined in recent years.
The research, published online Tuesday by the journal Science, shows that the Huanan Seafood Wholesale Market was likely the early epicenter of the scourge that has now killed nearly 6.4 million people around the world.
Experts said this “historic backsliding” in vaccination coverage was especially disturbing since it was occurring as rates of severe malnutrition were rising.

Novavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S.—and one that’s already available in Europe and multiple other countries.