Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe NowEli Lilly and Co. is pushing hard to gain a sales edge against two other drugmakers in the war against metastatic breast cancer. It is spending hundreds of millions of dollars to win over patients with an extensive advertising campaign.
The Indianapolis-based company, maker of cancer drug Verzenio, is blanketing airwaves with commercials that tout its drug’s track record in helping afflicted women live a little longer.
Metastatic breast cancer—also known as MBC or stage 4 breast cancer—is a tough, crippling disease without a cure that claims about 40,000 lives a year. The disease is the most severe form of breast cancer. Nearly three-quarters of all women diagnosed with the disease die within five years.
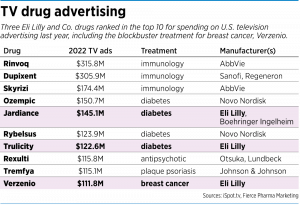
Last year alone, Lilly spent $111.8 million advertising Verzenio, according to ad-tracking specialist iSpot.tv, as reported by Fierce Pharma Marketing, an industry newsletter. That made Verzenio the 10th-most advertised drug in the U.S. by spending, up 60% from $70.1 million in 2021.
No other cancer drugs broke the top 10 list for advertising spending. (Lilly has two other drugs on the top 10 list—diabetes drug Jardiance, fifth-highest; and diabetes drug Trulicity, seventh-highest.)
Lilly declined to say how much it is spending on its Verzenio campaign, or to confirm the outside estimates. But it defended the use of the direct-to-consumer marketing effort.

“There is a need to educate about complex disease like breast cancer,” Monica Popp, Lilly’s associate vice president for U.S. oncology marketing, said in an emailed statement to IBJ. “We invest in these campaigns because of the importance of raising awareness about breast cancer and Verzenio as a potential treatment that [patients] can discuss with their physicians, in the hopes that we can help patients advocate for themselves.”
The drugmaker has produced about 10 TV spots for Verzenio since 2018, and two of them are currently running as part of a “Future Photos” campaign for women with metastatic breast cancer. It is also running two spots highlighting use of Verzenio for treatment of early breast cancer as part of its “Make Your Way” campaign.
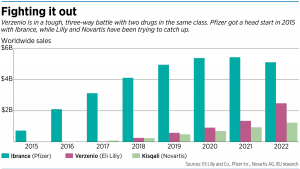
Lilly shows no sign of slowing its advertising push for Verzenio as it competes against Pfizer’s Ibrance and Novartis’ Kisqali in the war against metastatic breast cancer.
All three medicines belong to a class of drugs called CDK inhibitors, which work by blocking overactive enzymes that would otherwise allow cancer cells to proliferate.
Metastatic breast cancer is one the fastest-growing areas of medicine. Strategic Market Research, a New York City-based research firm, estimates the market for metastatic breast cancer treatment was $17.1 billion in 2021 and is expected to reach $41.7 billion by 2030.
Ibrance and Kisqali have also advertised heavily in recent years, but not at the same level as Lilly, and have never broken into the top 10 list of drug advertisements by spending.
The drugs are not cheap. List price for Verzenio or Ibrance is about $14,500 a month. For Kisqali, the price ranges from $6,000 to $15,000 a month, depending on the dosage. The actual price for all three drugs varies, depending on health plans and pharmacies.
Three-way fight

Some experts say the three-way advertising war is likely to confuse patients, as they try to figure out, with their doctors, which medicines are likely to help and what to expect from the side effects.
Common side effects for Verzenio, for example, are diarrhea, low white-blood-cell counts, anemia, nausea, headaches and tiredness.
“Competition can be good if it keeps prices down, but otherwise it can be confusing, because they all have different risks,” said Diana Zuckerman, an epidemiologist and president of the National Center for Health Research, a nonpartisan health think tank in Washington, D.C. “It’s rather impossible for the average person to make sense of the list of risks even if they read them.”
All three drugmakers have been conducting expensive clinical trials to try to show the cancer treatments are helpful for related breast cancer problems. That would allow them to talk up the drugs more extensively with doctors.
In 2021, Verzenio was the first CDK inhibitor approved as a so-called “adjuvant therapy”—or therapy after breast surgery to target remaining tumor cells—for an early stage of a common form of breast cancer known as HR-positive, HER2-negative.
Three months ago, the U.S. Food and Drug Administration expanded Verzenio’s label indication for use in combination with endocrine therapy for the adjuvant treatment of adult patients with an HR-positive/HER2-negative, node-positive, early breast cancer at a high risk of recurrence.
That expanded Verzenio’s eligible patient population in early disease. “This expanded approval will allow us to bring Verzenio to many more women and men with HR+, HER2-, high-risk early breast cancer in the curative setting—before patients experience recurrence, potentially to incurable metastatic disease,” said Jacob Van Naarden, CEO of Loxo@Lilly, the drugmaker’s oncology division, in a press release.
Last December, the FDA approved a label expansion for Pfizer’s breast cancer drug Ibrance. It said Ibrance could be used in combination with an aromatase inhibitor for newly diagnosed HR+/HER2- metastatic breast cancer patients regardless of menopausal status. (Aromatase inhibitors are an older class of drugs used in the treatment of breast cancer in postmenopausal women.)
The other drugmakers are pushing hard to expand their labels as well.
Previously, the combination of Ibrance and an aromatase inhibitor was limited to use in postmenopausal women. The expansion could allow Pfizer to market the drug to premenopausal women, who represent about 17% of the HR+/HER2- breast cancer patients.
But just a few months later, in March, Novartis had its own big announcement. It reported that its cancer drug, Kisqali, when used on top of hormone therapy after surgery, significantly reduced the risk of invasive disease recurrence, compared with hormone therapy alone for HR+/HER2- early breast cancer.
That, too, would expand the potential market for the drug, turning it into a “multibillion-dollar opportunity” for Novartis, CEO Vas Narasimhan said during the company’s first-quarter earnings call.
Jeff Legos, Novartis’ global head of oncology and hematology development, echoed that sentiment, saying that would widen the patient base for Kisqali substantially.
“We believe that our population is much broader and represents about twice as many patients who may now have the potential of benefiting from the CDK4/6 inhibitor,” he told industry newsletter Endpoints News.
“Novartis’ win is a direct threat to Eli Lilly’s rival drug Verzenio,” Fierce Pharma reported in March.
In short, the competition is turning into a series of claims and marketing boasts that only an expert is likely to understand. Perhaps that’s why the drugmakers turn down the technical language and turn up the emotional heartstrings in direct-to-consumer advertisements.

Latest Lilly commercial
Last month, Lilly launched its latest commercial for Verzenio, an upbeat, 60-second spot that encourages patients to look ahead, not just back. The commercial opens with a 60-something, gray-haired woman sitting on a couch, flipping through a photo album.
“Living with metastatic breast cancer means I cherish my memories,” she says in a voiceover. “But I don’t just look back on them. I look forward to the chance to make new ones every day with Verzenio.”
The camera zooms in to show a new section of the album, titled “Future Memories.” The pages scroll by, showing pictures of the woman at an alumni reunion, a backyard cookout, a New Year’s Eve party and other celebrations, with everyone wearing big smiles.
“Verzenio is proven to help you live significantly longer when taken with fulvestrant,” the announcer says. How much longer? According to small type at the bottom, women who take Verzenio and fulvestrant (an older drug for breast cancer) lived for a median of 46.7 months, versus 37.3 months on fulvestrant alone.
Some experts raised an eyebrow when asked about the “significantly longer” claim, given that the additional survival benefit of Verzenio is about nine months, compared to taking an older drug alone.
“When people hear ‘significant,’ they probably think an extra year or two of life, at least,” Zuckerman said. “For cancer drugs, living nine months longer is considered a meaningful benefit, unless the side effects—nausea, vomiting, diarrhea, exhaustion, etc.—make a person’s life miserable. Wouldn’t you rather have 37 enjoyable months instead of 46 miserable months?”
Indeed, the announcer spends nearly half of the 60-second Verzenio spot listing common side effects and warning patients to see their doctors immediately. (“Blood clots that can lead to death have occurred.”)
Lilly said its direct-to consumer marketing campaign has been successful “at raising awareness and helping patients feel more prepared for discussions about Verzenio with their physicians.”
Two national patient-advocacy groups, Breast Cancer Action and the National Breast Cancer Coalition, declined to comment about the competition among the three drugmakers or the effectiveness of the drugs. Nor did they comment about whether direct-to-consumer marketing was helpful.
Some breast cancer patients who are taking Verzenio acknowledge that the drug has powerful side effects, including diarrhea, but they take it on the advice of their oncologist.

Gitte Joergensen, 45, a research associate at the Connecticut Institute for the Brain and Cognitive Sciences, is taking Verzenio as a high-risk, stage 4 lobular breast cancer patient. She said before starting the drug, she asked her oncologist to write down its name.
“She did and then told me not to Google it,” Joergensen told IBJ. “And that’s the thing—everyone pretty much Googles it and knows about the side effects before they begin taking Verzenio.”
She said her Facebook group encourages patients taking Verzenio not to be afraid to talk about the diarrhea and how to deal with it.
“It’s by far the most popular subject on Facebook, and we all enjoy having a laugh about it as well,” she said.
The ads run only in the United States, one of the few countries to allow direct-to-consumer drug advertising. IBJ asked a few patients who live overseas to look at the ads on the website iSpot.tv for their reaction.
Debbie Donnison, 61, of Worcester, England, who was diagnosed in 2022 with stage 4 breast cancer, said she has read the package insert sheets carefully but was alarmed when listening to the announcer rattling through them in the TV spot.
“They sound terrifying without context,” she said. “…They say them as fast as possible whilst your brain is saying, ‘Hey, hang on a minute.’ I realize they don’t want to focus on them, though, and time is short.”•
Please enable JavaScript to view this content.